The Janik group applies the tools of computational chemistry to understand and design materials for alternative energy applications. In the battery area, we currently have two ongoing projects: 1) the computationally guided design of ionic polymers as battery electrolytes (collaboratively with Ralph Colby, Materials Science and Engineering) and 2) design of nanocomposite cathodes for Li-S batteries (with Donghai Wang, Mechanical Engineering).
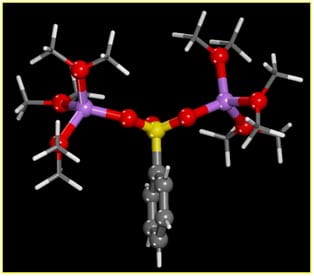
sulfonate-Li+ triple cation solvated
by ether oxygens representative
of a polyethylene oxide polymer backbone.
In the first project, the algorithms of quantum mechanics are used to evaluate the interaction between ionic groups, polymer backbones, and conducting ions. Computed interaction energies are input into conduction models to evaluate the relative conductivity of various ionic polymers (ionomers). In the second project, our computational work supports experimental work by the E-Nano laboratory of Donghai Wang to determine the optimal metal-oxide additives to enable cyclability of Li-S batteries. Metal-oxides are evaluated for their ability to adsorb and catalyze the redox cycle of lithium polysulfide compounds, therefore preventing them from dissolution into the electrolyte and eventual degradation of battery performance. Our laboratory also has active projects examining a number of heterogeneous catalytic systems, including electrocatalysis within fuel cells and hydrogen generation. We have experience developing density functional theory based approaches to electrochemical interfaces, and have applied these to electrocatalytic processes within proton exchange membrane fuel cells and direct borohydride fuel cells.
In the first image at left, Li cations (purple) interact with the oxygen atoms (red) of both the sulfonate ion (sulfur, yellow) and ether oxygens (represented by di-methyl ether molecules). This triple ion species likely represents the conducting species for Li+ transport through anionic polymers, with conduction occurring as a Li+ cation is transferred to another ion pair.
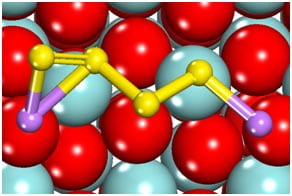
species formed during discharge at the
cathode of a Li-S battery, is adsorbed to the
surface of ZrO2.
In the second image at left, Li atoms (purple) interact with surface oxygen (red) atoms while sulfur atoms (yellow) interact with Zr atoms (turquoise). Adsorption of these intermediate species by added metal oxides can prevent dissolution into the electrolyte and eventual battery performance degradation.
